Last time we talked about the unusual properties of elemental nitrogen mostly and how stable it is. We only touched on a little of the fascinating and extremely complex chemistry of nitrogen, ONCE we can get it in a form other than the incredibly stable elemental form.
This time we shall remedy this, although entire graduate level texts have been written on the subject. Tonight we shall take a brief survey of the impact that nitrogen has on living organisms, industry, and a few other areas. We shall attempt to do this by looking at various oxidation states, and nitrogen has more than any other element.
The basic concept is that atoms can either donate or accept electrons from other atoms. When an atom donates electrons, it is oxidized, and when it accepts electrons it is reduced. Thus, chlorine bleach works because hypochlorite ion is a strong oxidizing agent and breaks up large, colored molecules to smaller, colorless ones.
Nitrogen can have eight oxidation states, including 5+ (having lost five electrons), 4+, 3+, 2+, 1+, 0, 1- (having gained an electron), 2-, and 3-. This makes for an extremely rich chemistry potentially possible, and nitrogen does not disappoint. How do atoms gain or lose electrons? Actually, they really do not except in solution, but this is a convenient bookkeeping fiction that allows us to account for electrons.
Apart from using electrical currents, atoms gain or lose electrons for the resulting molecule or ion to gain stability over the previous state. In first and second row elements, all atoms are more stable when either two electrons (for the first row) or eight electrons (for the second row) are around them. This is a result of quantum mechanical reasons, but was known long before quantum mechanics were developed. This is not always the case, but is very often the case.
Atoms can either share electrons equally or one atom can have greater electron density than another. The former case is usually when two or more of the same kind of atoms are involved, and the latter when two or more different kinds of atoms are involved. The degree to which electrons are more greedily held by a given atom is called the electronegativity of the atom. This scale, originally developed by the brilliant American chemist Linus Pauling, ranges from 0.7 to 3.98 (originally 4.0, but now more refined). Elements with low numbers readily give up electrons, elements with high numbers readily accept electrons, and elements with numbers in the middle can do either, depending on circumstances.
Nitrogen comes in at 3.04, and only three elements (oxygen at 3.44, fluorine at 3.98, and chlorine at 3.16) have greater electronegativities. Let us look at the very common nitrogen compound ammonia, NH3. Hydrogen has an electronegativity of 2.20, so we would expect for it to donate electrons to the nitrogen. It does, but a considerable amount of electron density remains around the hydrogens since the difference in electronegativities is only 0.84. Thus each hydrogen, a first row element, has some electron density with two electrons (each hydrogen shares electron density with nitrogen which has three electrons to participate) and nitrogen, a second row element has electron density with eight electrons (nitrogen has three valence electrons and a lone pair for a total of five), giving each atom the needed number for stability.
In our accounting system we would say that hydrogen has an oxidation state of +1 (being one electron short) and nitrogen has an oxidation state of -3 (having three extra electrons). As I said, this is just bookkeeping because nitrogen only has PART of the electron density from hydrogen. But it makes it easy for chemists to "push" electrons to make chemical reactions make sense.
Now let us look at nitrate ion, NO31-. This ion has an overall charge of negative one, as shown by the superscript, and must be associated with other, positive ions in the solid state to provide an overall neutral material (otherwise electrostatic repulsion would cause the mass to fly apart). Doing the accounting, we see that it is not really that easy like it is in ammonia. Here is a diagram showing the Lewis dot structure of nitrate.
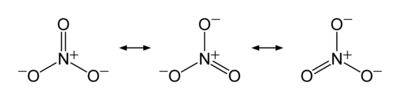
These resonance structures represent the three bonding situations betwixt three oxygens and one nitrogen. Note the double headed arrow: it denotes that none of the structures shown are reality, but that the actual result is a superposition of all three. Since all of the structures are identical except for which oxygen is involved (in this ion all of the atoms are in the same plane, what we call a trigonal planar arrangement) so that there are no geometrical differences, all three structures contribute equally. But where does the extra electron arise? It comes from either hydrogen (in nitric acid) or a metal ion (the ammonium ion also counts, as in ammonium nitrate).
Now we count electrons and assign oxidation states. Remember, oxygen has a higher electronegativity than nitrogen, so for our bookkeeping nitrogen is losing one or more electrons and oxygen is gaining it (or them). Nitrogen has, as an atom, five electrons with which to fool around, and oxygen has six. To get all three atoms "happy" (sharing eight electrons each) we have four atoms and 23 electrons, but we need 24. That is why the counterion contributes one. For hydrogen, ammonium, and the alkali metals, with lower (for hydrogen) and much lower (for ammonium and the alkali metals), they are "glad" to contribute a significant amount of their single, outer electron density to gain stability. That makes nitrogen have a 5+ oxidation state overall because all five of its outer electrons, including its lone pair, are involved in bonding with oxygen.
Nitrates are extremely important in industry and agriculture. They are mostly produced synthetically from Haber ammonia, but mining still occurs in some parts of the world, notably Chile. Nitrogen is essential for protein and nucleic acid formation, and nitrate is a soluble form that plants can assimilate readily. The champion for nitrogen content in fertilizer is ammonium nitrate, since both ammonium ion and nitrate are highly water soluble and easily taken up by plants.
In industry, nitrates are used as oxidizing agents for many processes, particularly explosives. Ammonium nitrate is used in blasting, usually mixed with fuel oil since it has more oxygen than in needs and the fuel oil contributes to the output. These explosives are rather insensitive to initiation and so are relatively safe to handle. Potassium nitrate is a key component in black powder, now a niche use.
Cured meats use nitrate to help preserve it, but there is more to it than that. This brings up nitrite ion, NO21+, still with an overall 1+ charge.
Here is a diagram of this ion. Note that only two oxygens are bonded to the nitrogen and that the ion is not as symmetric as the nitrate ion. Not shown in the diagram is the lone pair of electrons on the nitrogen, and they are located above the nitrogen. In this case only three electrons enter into bonding with oxygen, so the oxidation state of the nitrogen is 3+.

Some nitrate is reduced to nitrite when meat is treated with nitrate, and it is really the nitrite that does the heavy lifting in meat preservation (other then the large amount of regular salt in many cured meats). It turns out that nitrite is a specific inhibitor of Clostridium botulinim, the bacterium responsible for the most severe form of food poisoning, botulism. The word botulism comes from the Latin botulus, "sausage". Nitrites also give off some nitric oxide (we talked about that last week) and it combines with the heme group on the myoglobin (one of the major proteins in muscle) and gives ham that nice pink color.
Those of you who barbeque may have noticed a pink layer in meat that has been smoked. This is also caused by nitric oxide, this time produced by the combustion of some nitrogenous compounds in the wood used to produce the smoke. Nitrite is fairly toxic and in the US residual nitrite in commercial meats is limited by regulation to 200 parts per million or less. Use caution when home curing meat and do not exceed the recommendations on the curing product. There is another problem with nitrite and that it can combine with amino acids in proteins at high temperatures to form nitrosamines, known human carcinogens. So be sure not to burn your weenies or blacken your bacon when you cook them.
Actually, nitrites are not necessary for bacon, hot dogs, and other "cured" meats that are kept under refrigeration. Those meats are not really cured at all, but rather flavored with dilute amounts of curing materials and usually smoked. We enjoy them because for generations we have become accustomed to the flavor of the curing agents. Meat that is truly cured is quite dry and very salty, but it will keep for months or years without refrigeration. I have noticed on TeeVee adverts the past couple of weeks that a major producer of lightly treated meat products has introduced a line of them without nitrate nor nitrite that they call "uncured". They are still flavored with salt, some sugar, and smoke but will not produce nitrosamines when overcooked.
Let me see if I can think of an example of each oxidation state of nitrogen without looking any up in a book. We already did 5+ and 3+, so let me think of a 4+ one. Sure enough, it is nitrogen dioxide (NO2), an important air pollutant formed when atomospheric nitrogen is partially oxidized, as in internal combustion engines. It has a very interesting structure, since it is a rather stable free radical (having one unpaired electron). Molecules with a single unpaired electron are called doublets because of their spectral characteristics. Here is a sketch that I made:

Each oxygen has six valence electrons to use to make the molecule and the nitrogen only five. That gives 17 electrons overall, and these resonance structures come as close to eight electrons around the nitrogen as possible, with seven. The oxygens both have eight. Many molecules with unpaired electrons are colored, and nitrogen dioxide a brownish red.
For nitrogen in the 2+ oxidation state, nitric oxide (NO) about which we spoke last week is one. Here is a sketch of its structure.
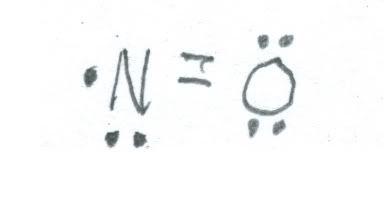
Unlike nitrogen dioxide, nitric oxide is colorless. It is also highly reactive, forming nitrogen dioxide on contact with air. It is also a doublet. Please refer to the comments last week about uses for nitric oxide.
An example of nitrogen in the 1+ oxidation state is nitrous oxide, (N2O) about which we also spoke last time. There are two ways to draw valid structures, but only one set fits with experimental evidence. Here are the two possibilities:
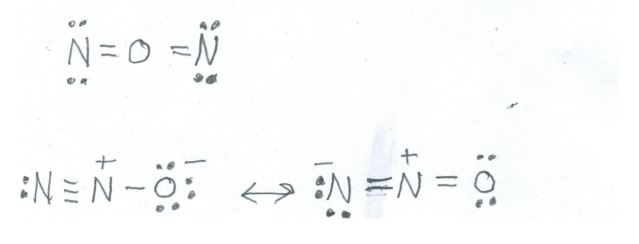
The single structure at the top is a perfectly good Lewis dot structure, but spectroscopic data on nitrous oxide indicate that the molecule is less symmetric, so the only structure that fits the facts is the resonance structure below. Each nitrogen contributes five electrons and oxygen six, so there are 16 electrons to place. Sure enough, nitrous oxide is an even numbered species, fairly stable, and colorless.
Nitrogen in the 0 oxidation state is easy. Elemental nitrogen, with a structure given last time, fits the description.
Now, for nitrogen in the negative oxidation states it is necessary for it to bond with atoms of lower electronegativity. The thiocyanate ion is kind of sort of an example of nitrogen in the 1- state. Here is a sketch:
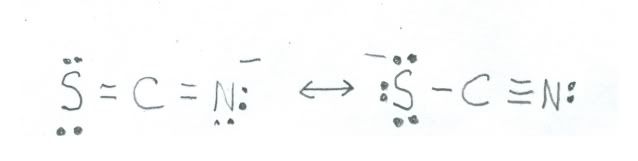
The resonance structure on the left shown nitrogen in the 1- state, but the one on the right has it in the 0 oxidation state. The one on the left is the major contributor, because sulfur has an electronegativity of only 2.58, so in comparison with nitrogen is much less suited to carry the negative charge. Chemistry can get sort of complicated!
OK, I struck out trying to think of nitrogen in the 2- oxidation state. I bet that someone out there can supply an example!
We already talked about ammonia, where nitrogen is in the 3- oxidation state last time. There is no reason to repeat that.
Hey, not bad for coming up with examples for seven of the eight oxidation states for nitrogen off of the top of my head! Chemistry is a love for me.
The reason for going to the trouble to give examples of the different oxidation states of nitrogen is that because of the facility of going from one state to another nitrogen is uniquely suited to enter into reactions that are extremely important to biological (and industrial) processes. Often it can go from one oxidation state to another, and in a final step be returned to the original one. Without this ability, life as we know it would be quite impossible.
Well, you have done it again! You have wasted many more einsteins of perfectly good photons reading this gaseous piece. And even though Romney realizes that he was blowing a dog whistle rather than making a lighthearted joke when he let the crack about no one asking for his birth certificate when he reads me say it, I always learn much more than I could possibly hope to teach when I write this series, so please keep comments, questions, corrections, and other feedback coming. Tips and recs are also welcome.
I may or may not be here for the entire Comment Time tonight. The Girl has not been feeling very well today, but she yet may give me a call to come and visit. If so, you know why I went silent, but I shall be back later. Remember, no science or technology issue is off topic in the comments. I shall return tomorrow evening around 9:00 PM for review time unless I do not (wink, wink, nudge, nudge!).
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at
The Stars Hollow Gazette,
Docudharma, and
firefly-dreaming


