The idea of carbon capture and sequestration (CCS) as a means of reducing CO2 emissions or even drawing down ambient levels as a means of mitigating climate change has been percolating into the public awareness more and more. This is Climate Week, Copenhagen is coming up in a couple of months, so it seems like a good time to look into CCS in a little more detail. This will be an overview of the science involved and technologies required.
"Carbon" here of course refers to carbon dioxide, the result of burning of fossil fuels, biomass, or biofuel, such as ethanol or methane. Today CO2 comprises some 387 parts per million or 0.0387% of the atmosphere. "Capture" refers to isolating CO2 from the rest of the atmosphere, and "sequestration" to sticking the captured carbon someplace not in the atmosphere.
An excellent overview, getting slightly dated, is the IPCC's technical report available here. The figures below are taken from that publication.
Capture: the Problem
Here's the basic issue. Imagine a volume of air. Out of every million molecules in that volume, only 387 will be CO2. Somehow you need to find and isolate those CO2 molecules, and only those CO2 molecules. For those more visually oriented:

The flask of water represents air, the blue dye CO2. The problem is to take the blue out of the flask and capture it in the little beaker.
Intuitively you know that's some trick; on more quantitative grounds you're fighting entropy. Thermodynamically you are going from a mixture to two unmixed quantities. Going the other way is of course very easy -- the solutions would mix together pretty much immediately. This tells you that the total mixture is thermodynamically favored, which means you have to apply energy to separate the mixture. The more spontaneous the mixing, the more energy required to separate the mixture. Releasing CO2 into the atmosphere, whether you're burning coal, gasoline, wood, or whatever, is VERY spontaneous. If you get nothing else from this post at least understand this. The hope is that the additional energy cost associated with CCS is not prohibitively high, not that we can make the energy cost go away. Here's a figure from the IPCC CCS report:

Note that "CO2 avoided" will always be less than "CO2 captured" -- the idea is to minimize that difference.
So what is the magnitude of the problem we're looking at? How much carbon do we need to sequester? There is no good consensus on what a desirable level of CO2 is, only that we're past it. A compromise between what we'd ideally like and what might be feasible seems to be converging to about 350 ppm (350 molecules CO2 out of 1 million molecules air), about what it was in 1987. We are currently at 387 ppm and climbing. (If you don't want numbers feel free to ignore the blockquoted stuff).
Let's run some numbers: The mass of the atmosphere is about 5.15 x1018 kg. The molecular weight of air is 28.97 g/mol, so that's about 1.78 x1020 moles of air (a very little bit less since the molecular weight does not include water vapor). Out of every million moles, 387 are CO2, or 6.88 x1016 moles CO2. CO2 makes up 99% of atmospheric carbon, and we did not include water vapor in our molar estimate; on the other hand CO2 is some 7 ppm or so lower above the troposphere (rate of mixing is slower than rate of emission), so the errors roughly cancel and we'll ignore the second order corrections for this purpose. At 12 g/mol that's about 8.26 x1017 g = 826 billion metric tons = 826 gigatons = 826 Gt carbon.
350 ppm is 750 Gt C, so if carbon sequestration were to be the only method for reducing atmospheric carbon, we would need to remove 76 Gt C. In practice, we would be supplementing natural uptake processes such as primary production (growing things) and ocean uptake.
What's that in more normal numbers? The density of crude oil ranges from 800-1000 kg/m3, depending on grade. Let's use 900 kg or 0.9 ton. The carbon content of crude also depends a little on grade, but is somewhere around 85% by weight. So 76 x109 ton C/(0.85 x 0.9 ton C/m3) = 99 x109 m3 crude. There are 264.17 gallons/m3 and 42 gallons to the barrel, so that's 624 billion barrels of crude oil equivalent, around 20 years worth of global crude oil consumption. That's what we're up against.
Because atmospheric CO2 is so dilute most efforts involve capturing CO2 from exhaust stacks, where concentrations are at least 10-15% (100000 to 150000 molecules CO2 per million molecules exhaust gas) and sometimes more. This is feasible for power plants but not for mobile sources, such as cars. There are four general approaches:
- Capture post-combustion
- Capture pre-combustion
- Burning in pure oxygen (oxyfuel combustion).
- Sequestration at time of capture.
The first three can be summarized in this figure ("Industrial processes" are those using fuel directly as opposed to those that use electricity drawn from a power plant):

It should be noted that capture technology is much more mature than sequestration technology. The issue is not whether we can do it, but what is the cost in energy and money.
Post-combustion capture
This is just what it sounds like: coal or natural gas in burned in a furnace or turbine, and the CO2 captured from the exhaust. There are three general approaches here:
Cryogenic separation
Basically you lower the temperature until CO2 condenses out. Because the components of air are not ideal gases they condense out, and they happen to condense out at different temperature. To liquefy O2, for instance, you need to chill air down to 90K (-298o F); to liquefy N2 you need to chill to 77K (-321o F). CO2 condenses out at a relatively balmy 195K (-109 o F).
Cryogenic separation works quite well and is a well established and widely used technology. But it costs a lot of energy. As a consequence, this approach is only feasible for exhaust treatment of stationary power plants, where CO2 may make up 10-15% of the exhaust gas volume. IOW, to capture 10-15 CO2 molecules, you only need to chill 100 exhaust gas molecules; in the free atmosphere, to get 10 CO2 molecules you'd need to chill 25900 gas molecules.
Chemical scavenging
The idea here is to bind CO2 to something else, either in solution or on a surface, where it then can be released in a controlled space (storage vessel). This approach is in fact the leading candidate for carbon capture (particularly the basic scavenging form), we know it works, but there's still a fair bit of research going into it.
Basic scavenging
CO2 dissolves in water to form a weak acid, H2CO3:
CO2 + H2O <--> H2CO3 <--> H+ + HCO3- + CO3=
If we make the solution weakly basic, typically with something like ethanolamine or similar, we shift the equilibrium to favor HCO3-, which efficiently scavenges CO2 from the waste stream (we could make it strongly basic and favor CO3= but that makes it harder to release the CO2). Now we have scavenged CO2 from the exhaust stream but we're left with billions of gallons of HCO3- laden water per power plant. Consuming enormous amounts of water per plant is not a realistic option in terms of energy, water, or money, so we need to recycle the water. The easiest way to do that is to acidify it, shifting the equilibrium back to CO2. Clearly this would be done in sealed areas vented to a pipeline or large tanks, not to the atmosphere. The main energy cost here is in basifying and acidifying the solutions. We also have to ensure that the exhaust gas is well scrubbed of sulfur and ideally NOx: sulfur forms sulfuric acid, NO2 forms nitric acid, both of which will force greater use of the base (to ensure basic conditions) and are difficult to extract from the base. Most of the NOx will be in the form of NO at this stage so sulfur is the bigger problem.
Solid scavenging
This is similar to the above, only CO2 is scavenged with a solid instead of a liquid. There are two general approaches. One involves reacting CO2 with a solid such as CaO:
CaO + CO2 <--> CaCO3
The CaCO3 would then be taken to a storage area and "recharged", driven back to CaO. This is done by heating, and is basically the process used to make cement.
Scavenging can also be done on porous minerals known as zeolites. These are compounds with enormous surface areas which are great at adsorbing gaseous compounds (or liquid, come to that: Adsorption is takeup on a surface, absorption throughout the bulk). The zeolite or whatever you're using is then recharged in a storage area, perhaps by thermal desorption though more likely by pressure swing desorption. Again, a clean gas stream helps to extend the life of the adsorber.
The problem with both basic and solid approaches is the immense amount of CO2 that must be scavenged. Regeneration of either the base or the solid species as well as transport from capture to release and back form the bulk of the added energy costs.
Membrane separation
This approach uses the fact that you can design membranes to be preferentially permeable to one species and not another. That is, forcing a gas volume against this membrane will allow a target species, in this case CO2, to pass through but not anything else. This is also well established technology, but not for CO2.
Capture precombustion
OK, I know this sounds odd. The thing to remember is that what's being burned here is hydrogen, not hydrocarbon. The trick is to break up the hydrocarbon to form syngas, CO + H2, in a process called gasification. There are two basic approaches here, steam reformation and partial oxidation (POX).
Steam reformation
Hydrocarbons will react with steam at high temperatures in the presence of a catalyst to form syngas. Steam reformation of natural gas (methane) is in fact how hydrogen is commercially made:
CH4 + H2O -> CO + 3 H2
The H2 is then used to upgrade low grade oil, tar sands, fed to fuel cells, used to make ammonia (and consequently fertilizer), etc.
For power plant applications, such as Integrated Gasification Combined Cycle plants, the CO is then reacted with more water vapor in the water gas shift reaction (WGS):
CO + H2O -> CO2 + H2
The CO2 is scavenged by one of the methods described above and the H2 fed either to a gas turbine or a fuel cell (a fuel cell is an electrochemical cell, just like a battery, only the fuel cell has external inputs and outputs. Batteries are self contained, which is why they run down.)
If it seems to you like this consumes a lot of water you'd be right.
Partial oxidation
This involves burning the hydrocarbon under oxygen starved conditions. If you burn a hydrocarbon to completion (full oxidation) you get CO2 and water. Under oxygen limited conditions you get incomplete oxidation and you're left with CO + H2, syngas. This is still an exothermic reaction (not nearly as much as full oxidation, obviously) and you get energy out of it. What you don't get is nearly as much hydrogen as in steam reforming, since you're not providing a hydrogen source.
Anyway, once you have your syngas, do the water gas shift, separate out the CO2 for sequestration, and feed the H2 to a gas turbine or fuel cell. A fuel cell has better thermodynamic efficiency than a gas turbine, but the waste heat of the turbine (and process heat if you're using POX) can be used to drive a steam turbine, improving the total thermal efficiency of the IGCC plant.
Note that gasification (either method) can use a range of organic feedstocks, from coal to petroleum to natural gas to biomass to organic waste, including some plastics. The greater the H:C ratio in the feedstock, however, the more energy per CO2 you get (same for regular combustion). Methane H:C = 4, petroleum = 2, coal depends on grade, 1 or a bit less is a reasonable average: clearly coal is the least desirable feedstock (it also typically has the most undesirable crap in it -- sulfur, arsenic, uranium, mercury, etc.)
Syngas, BTW, can instead be used to make liquid fuels via Fischer-Tropsch and related reactions.
Oxyfuel combustion
Here the fuel is burned in "pure" oxygen. Since the only reaction products are CO2 and H2O, and H2O is pretty easy to isolate, CO2 capture is much easier. In practice, the flame temperature in pure oxygen is extremely high, and you would take that down by recycling some of the exhaust back into the burner, as CO2 and H2O are not going to burn further (so it's not really pure oxygen). High flame temperatures will generate too much NOx from the residual N2 that is bound to be in the input stream; it is also more demanding on equipment.
The cost here is of course the separation of air into O2 and everything else. Oxygen is usually separated by chilling -- but its boiling point is much less than that for CO2 (90K vs 196K). Oxygen does make up 21% of the atmosphere, however, which helps a little. Oxyfuel combustion is still in the research phase and it is not clear whether it will ever be competitive.
So what is the energy tax associated with the various carbon capture schemes? It's a little hard to say right now, since we don't have real world operational data, but it will probably look something like this:

Not surprisingly it takes more work to clean up CO2 from coal than from natural gas. We might get improvement in these numbers as technologies improve; the numbers might get worse if we have issues with scaling or unexpected costs or issues arise. All we can say is that CCS will entail an energy cost.
===========================================
Sequestration
OK, somehow you've captured CO2, now what? Basically you need to stick it somewhere where it won't come back in the foreseeable future. There are three main options:
- Marine sequestration
- Geological sequestration
- Use CO2 for industrial processes
Marine sequestration
The surface ocean may be supersaturated with respect to CO2 (actually the aragonite form CaCO3) but the solubility increases dramatically at high pressures and low temperatures. The deep ocean already contains some 38000Gt C and remains undersaturated: it can easily handle all of the CO2 we've produced or will produce from fossil fuel combustion with capacity to spare.
What it can't do is hold it for very long. Oceanic turnover times are on the order of a few centuries, and most of the deep ocean carbonate is in equilibrium with a preindustrial atmospheric concentration of ~280 ppm. As the ocean turns over that dissolved carbonate will come back up and outgas back into the atmosphere, further acidifying the surface ocean on the way up. Maybe we'll have figured out a better way to deal with both atmospheric CO2 and ocean acidification before it comes back up; then again, out of sight, out of mind, and maybe we're just loading a carbon bomb.
I talk about this more here and here.
Geological sequestration
This approach buries CO2 in rock formations. There are a number of possibilities here.
Salt domes
Salt domes are giant plugs of salt that form from geological burial of evaporites (the salty minerals left on evaporating sea water). Salt is less dense than most rocks, and tends to collect and migrate upward in huge domes until they reach impermeable caprock. The salt itself is also impermeable, so stuff which can move and dissipate (eg, petroleum, natural gas) are frequently found associated with them. Our Strategic Petroleum Reserve is largely held in salt domes.
In principle this would work. We do have to be careful of leaks, especially at the injection point. This is easy to do for maintained injection points, but we are looking to sequester CO2 indefinitely, so we cannot take this for granted. Salt domes are common around the world, many in geologically stable areas.
Enhanced oil recovery/coal methane extraction
When an oil well is first drilled, the pressure of the overburden is relieved at the drill hole and care must be taken to prevent blowouts. This pressure is what drives oil out of an oilwell. However, as the well becomes depleted, the pressure drops, and eventually at equilibrium the well stops producing, even though there are still significant quantities of oil remaining. The options are either to pump it out or force it out, by injection of a gas or sometimes fluid. Using CO2 as the gas would replace the buried oil with CO2 (actually a fair amount of CO2 ends up in the oil, which helps reduce viscosity and improve flow, but would have to be reinjected for sequestration).
One can do something similar for extraction of natural gas from unmineable coal seams.
The advantage here is that we are using a waste product from one purpose to so something we're already doing more efficiently. The disadvantage is that that something is digging up more fossil fuels -- the stuff that's causing this problem in the first place, and the reason we're expending so much effort to alleviate -- than we might otherwise be able to do. As far as climate mitigation is concerned, this is a Red Queen's Race of the demented variety.
Leaks are a potential problem, especially in fields that have been hydrofractured. However, that depends on the rock and possibly the proppant. Even in the absence of leaks, overinjection or the fracking process itself may cause earthquakes, which even if not destructive themselves may cause leaks. To date some 100 Mt CO2 have been used for EOR and so far leakage has been near zero. Whether that will be the case for the indefinite future is not known, of course. Nor is the leakage rate of more aggressively fractured reservoirs (such as the Marcellus Shale or the Barnett Shale -- bothe of which are actually natural gas reservoirs) well known should they be used for CO2 sequestration.
Geological sequestration (not necessarily involved with EOR) may also increase the acidity of groundwater if the injected CO2 comes in contact with it.
Silicate reservoirs
A number of different types of rock react spontaneously with CO2 to form carbonate rock. This occurs naturally on geologic timescales, and the end product is as stable a form of carbon as exists on this planet. Probably the most common rocks suitable to the purpose are olivine and serpentine:
Olivine: Mg2SiO4 + 2 CO2 -> 2 MgCO3 + SiO2
Serpentine: Mg3Si2O5(OH)4 + 3 CO2 -> 3 MgCO3 + 2 SiO2 + 2 H2O
Both are very common rocks. Other common rocks that would work include pyroxene, wollastonite, and plagioclase feldspars. The difficulty here is that you want a good surface to volume ratio, so you will likely need to fracture the rock in the sequestration area. Leaks (unless huge) are not really an issue here as the CO2 will be taken up into a solid. The rate of injection needs to be carefully tailored to the porosity and crack structure of the rock reservoir: too fast and you will glaze the rock with carbonate, preventing the uptake of more CO2. You don't actually need silicate rock to do this, metal oxides such as CaO and MgO would work just fine -- but silicate rock is very common and metal oxides are not.
In principle mineral carbonation will provide a geologically stable form of carbon sequestration -- whether it will ever be feasible on anything like the scale required is not yet known.
Using CO2 for industrial processes
It is possible to "recycle" some of the waste CO2 for industrial processes. CO2 is used for a range of products including urea manufacture, methanol, various carbonates, fire suppressants (the use of which just releases it back to the atmosphere), etc. The scale of this is nowhere close to the scale of the problem, however. Timescales are also short: for methanol, eg., the lifetime of sequestered carbon is typically months to years; for a "long-lived" reservoir such as polyurethane, maybe a few decades.
So how much can we reasonably sequester? Sticking with known techniques (ie, leaving out mineral carbonation) here are some low/high estimates for global reservoirs in Gt C (remember to get to 350 ppm by CCS alone we need to sequester 76Gt + carbon cost of CCS):
Oil/gas fields: 185/245
Unminable coal seams: 1/50
Deep salt reservoirs: 300/3000
Note that DOE is rather more generous with coal seams and salt domes, more conservative with oil and gas fields (their numbers appear to be Gt CO2, so divide by 3.67 to get Gt C). The deep ocean can take up as much as we can possibly make, just not for long, and with potentially disastrous consequences to marine life.
There is also an ongoing cost of monitoring and reservoirs, verifying storage, and dealing with any leaks should they occur.
===============================
Transport
All of the scenarios laid out above require capture of CO2, transport to a reservoir, and storage in that reservoir. Transport costs are nonnegligible and require energy input, whether that involves diesel fuel for trucks or trains, bunker fuel for ships, electricity for pipeline pumps. The first two are almost certainly fossil fuels, since we have no way of making renewable liquid fuels sustainably in anything like the quantities required; in practice, the latter will also likely be coal or gas-fired.
Except for very short distances where flexibility is required, trucks are easily the least efficient. The most efficient (and least flexible) is pipeline. Pipelines typically run at high pressure (up to 2600 psi -- PDF alert), and compression of gas is also an energy cost. The gas must be dry, as dissolved CO2 forms an acidic solution which is corrosive. Leaks are also a potential issue, as CO2 is heavier than air and acts as an asphyxiant, and a major leak can be hazardous to nearby low-lying areas (this can also happen naturally, BTW). That said, pipeline technology is well established, and most failures are preventable (as in, don't skimp on maintenance). At present we have enough pipeline to transport some 50 Mt CO2/yr, which, given the scale of the problem, is not very much.
=================================
Sequestration at time of capture
It is also possible to sequester CO2 at time of capture. The main candidate for this is industrial mineral carbonation, which is chemically identical to the silicate reservoir: react CO2 with silicate or metal oxide rock to form stable carbonates.
The appeal here is that the end product is known to sequester carbon on geological timescales, provided you keep the carbonate away from rain (in which case it will dissolve and form HCO3-). Unlike the use of silicate reservoirs you can control the process and not worry about issues like porosity of the reservoir.
The downside is that this requires hard rock mining, milling, and transport. Unlike base or sorbent based chemical scavenging you cannot regenerate the chemical reactant: whatever you use will be lost for as long as the carbon is sequestered. That is, you need a constant input of processed rock. There is also the environmental cost of mining. So far this is easily the most expensive and potentially among the most permanent of sequestration techniques.
There are a number of other possibilities as well, some speculative, such as artificial photosynthesis. Real photosynthesis using algae, which has very high rates of growth, is an area of real interest. There has been a fair amount of research for the use of algae to make biodiesel -- technically it works, but economically it does not (I've heard cost estimates as high as $100/gallon, though DOE estimates 2x the cost of petrodiesel). It is difficult to maintain high production, algal strain purity is difficult to maintain, etc. Some of these issues are specific to the type of biodiesel they were trying to produce -- fatty acid ester biodiesel. For the purposes of CO2 uptake strain purity is not nearly as stringent an issue, which helps with other issues like reactor design (if you're going to make biodiesel Fischer Tropsch gives you a better product than FAE and does not require high lipid content or alcohol for esterification). But as a living thing it will always be far harder to optimize than industrial processes. In any case, the algae could then be pyrolized to form either biochar or syngas. Biochar appears to sequester carbon effectively on at least millennial timescales, and appears to improve soil structure and health as well. Whether this is economically feasible is not known, however.
Let's wrap up with brass tacks: what's all this going to cost? Again, we have no operational numbers, only estimates. That said, here's a recent estimate:
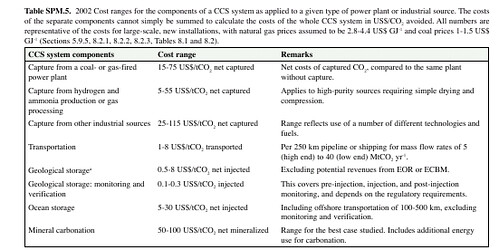
Capture is easily the most expensive part, but at least for cryogenic and chemical scavenging separation, the best understood. Separation involves fighting the Second Law, and decreasing entropy here requires a greater increase elsewhere -- there is no way around that. The consumption of carbon to remove carbon smacks rather of the ridiculous, but realistically we cannot stop use of fossil fuels cold turkey (not without massive dislocation and food and health shortfalls) and CCS will have to be a part of the suite of technologies used to wean us away from fossil fuel use. We have to accept that there is significant cost involved, we have to realize that this cost is one we've already started to pay, although mostly we've simply transferred the cost to the future to be paid with interest. That is, the cost is presently externalized and making itself manifest through climate change. Internalizing this cost provides a more realistic cost of fossil fuel consumption, which is necessary if we are to make informed decisions about energy production and use.


